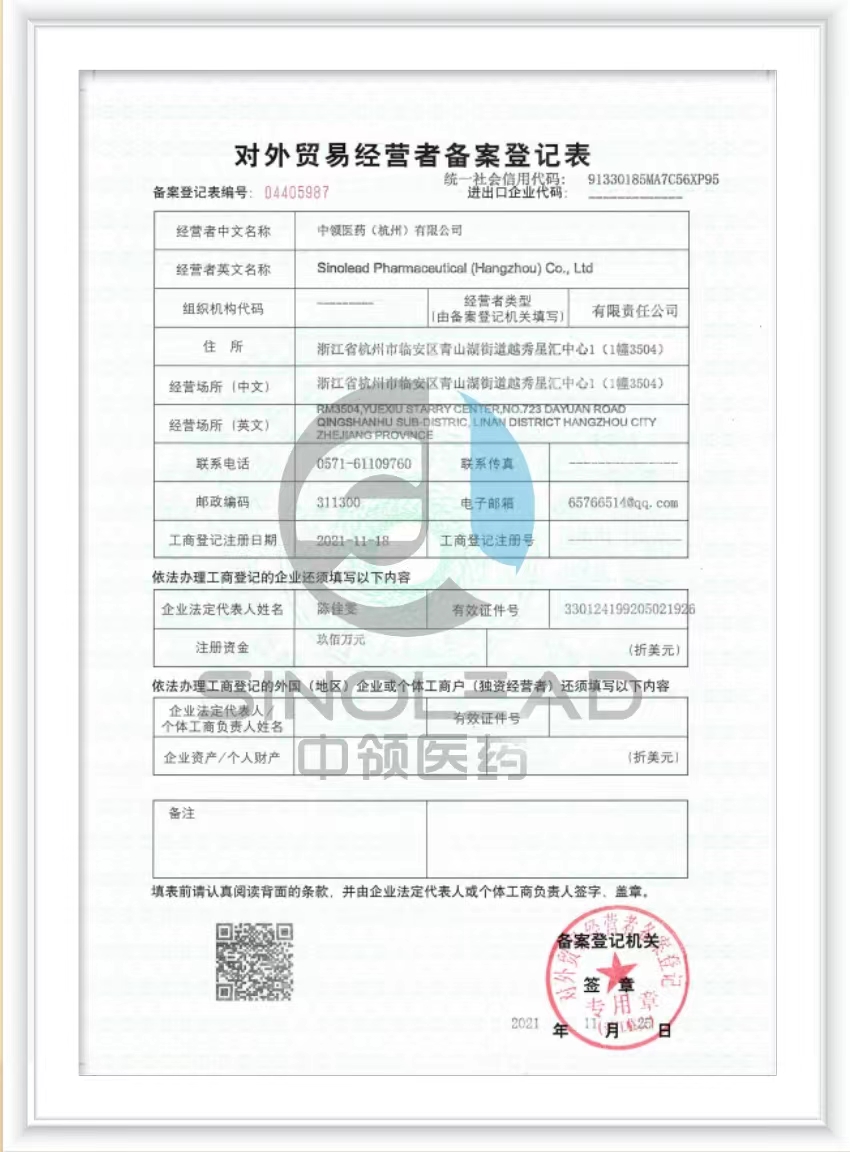
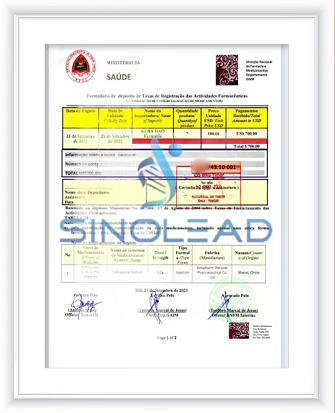
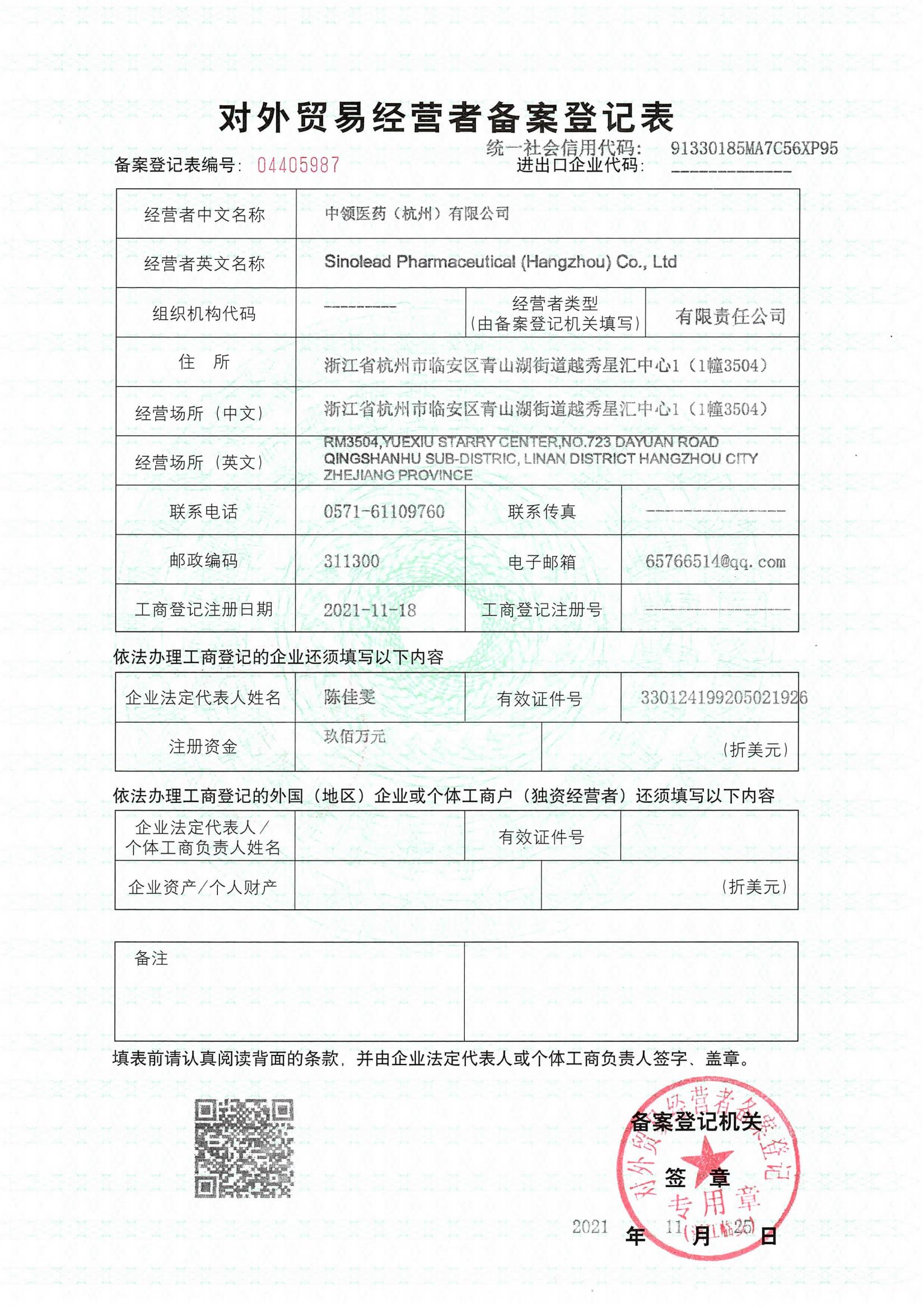
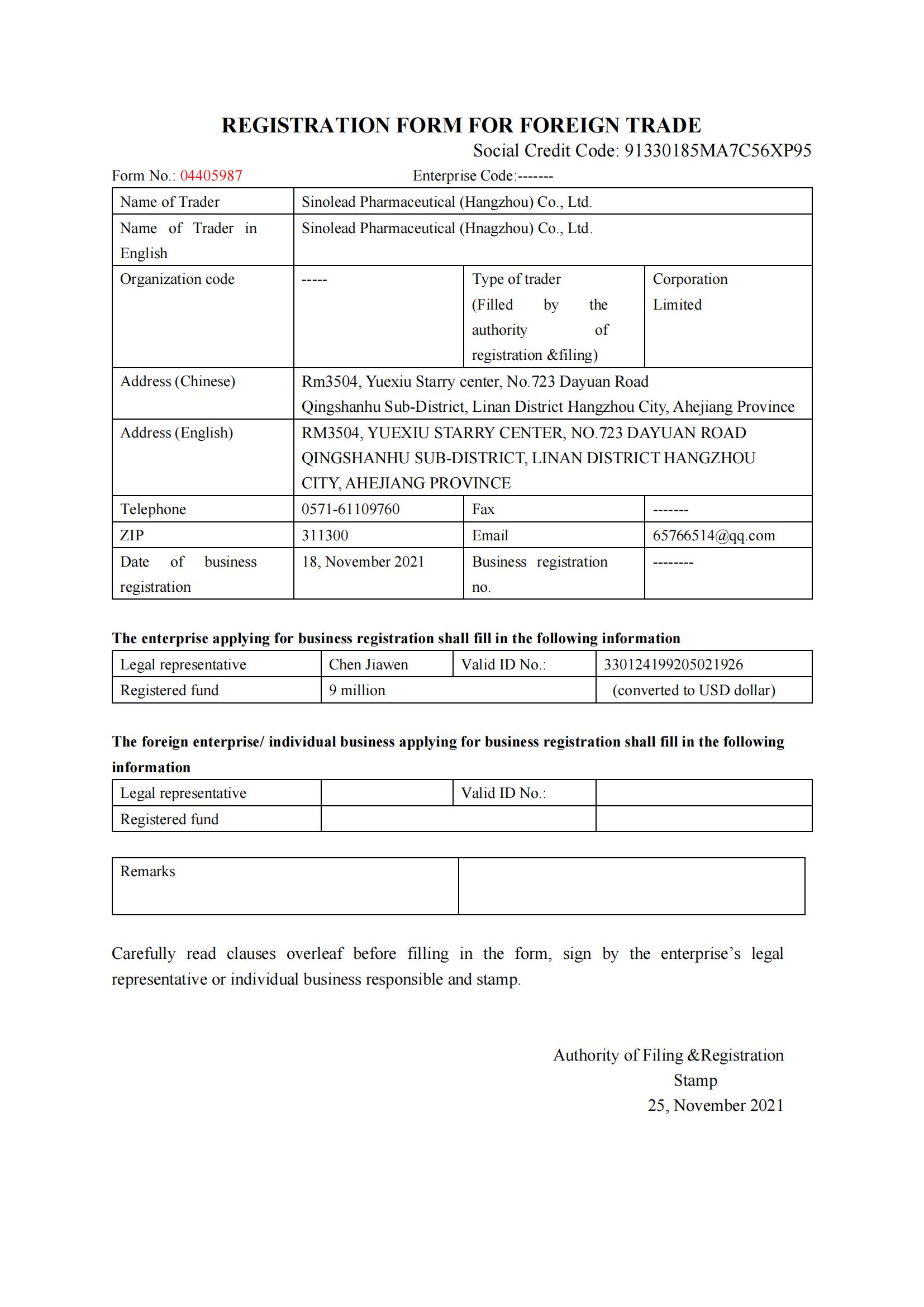
- Product Dossier in CTD/ACTD
- GMP Certificate
- Manufacturing Licence
- COPP(Certificate of Pharmaceutical Product)
- FSC( Free Sale Certificate)
- CCPIT certification
- Embassy Notarization
- Other documents based on request.
The terms, conditions, and specifications of the Sinolead-Client relationship are clearly stated.
Packaging Design
Sinolead begins preparing all necessary documents according to the importing country's regulations and requirements.
Factory Inspection
Some countries require a facility inspection by their MOH officials, unless previously inspected, in order to complete product registration.
Samples are required by most countries for testing in order to complete product registration.
Manufacturing Agreement
Dossier Preparation
Packaging is designed according to the client's requirements. Excellent packaging is one of the most important aspects in the marketing of our products.
After all documents have been prepared and samples produced, the healthcare products are submitted for approval.
Sample Production
Registration Subimission